

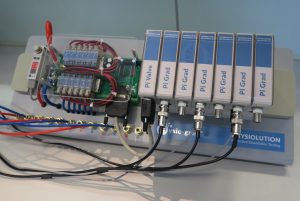
The pH value of a hydrogen carbonate buffer is the result of a complex and highly dynamic interplay of the concentration of hydrogen carbonate ions, carbonic acid, dissolved and solvated carbon dioxide and its partial pressure above the solution. The complex equilibrium between the different ions results in a thermodynamic instability of hydrogen carbonate solutions. The thermodynamic instability of hydrogen carbonate buffers offers the unique opportunity to simulate the dynamic intraluminal pH changes in the human small intestine and colon. In order to do so, both processes, the acidification as well as the alkalization of the buffer system require continuous and dynamic adjustment. For this purpose we developed a novel device the pHysio-grad® that enables a biorelevant simulation of intestinal pH gradients without changing the ionic strength of the solution.
The pHysio-grad® device:
- can precisely monitor and adjust the pH value of commonly used hydrogen carbonate buffers
- enables a precise pH adjustment in the range of pH 5.5 to 8.3 with a maximum deviation of ± 0.02 pH units
- utilizes pure CO2, mixtures of CO2 with other gases, inert gases such as air, N2 and He as titer gases
- is characterized by sufficient robustness, good performance stability as well as a highly dynamic pH adjustment
- can precisely simulate desired pH gradients within the physiological intestinal pH range
- can be used in various pharmacopoeial and non-compendial dissolution test devices

