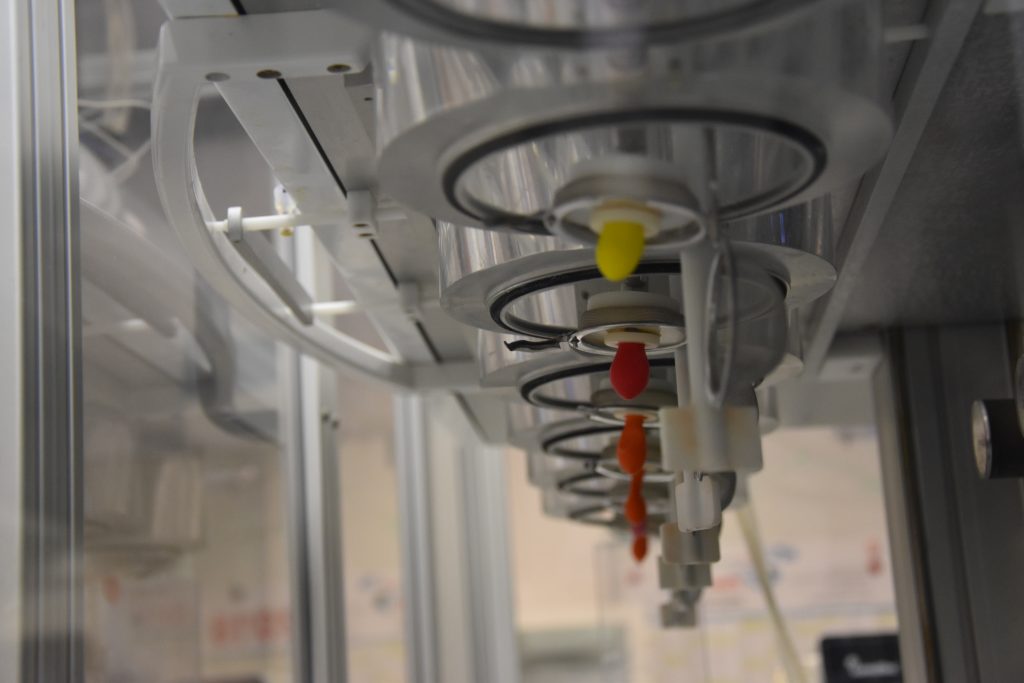
The bio-relevant test devices developed by Physiolution’s realistically simulate the mechanistic and physico-chemical conditions that act on dosage forms during their gastro-intestinal passage and enable the evaluation of their biopharmaceutical safety and reliability.
The dissolution stress test device enables a biorelevant simulation of the physiological mechanical stress that may occur during GI passage of a solid dosage form. The device simulates essential physiological stress parameters including discontinuous dosage form movement and GI motility forces using physiology-based test algorithms as well as the physico-chemical conditions.
The key factors of physical conditions of the GI passage that may affect the drug delivery behaviour of solid oral dosage forms considered by the bio-relevant dissolution stress test device are as follows:
- the discontinuity of the movement of dosage forms along the GI tract with peak velocities of up to 50 cm/s
- the GI motility in the form of sequences of mechanical contractions with an intensity of up to 300 mbar
- the intermittent contact of the dosage form with GI fluids
- the physiological volume changes of liquids and their flow patterns such as gastric emptying rates specific for different prandial conditions and patient groups
- the intragastric temperature gradients characteristic under fasting conditions
By this, the stress test device can demonstrate the conditions of GI transit in a biorelevant way. During the tests the dosage forms are exposed to sequences of agitation including movement and pressure fluctuations as well as phases of rest. The intensity, timing, and duration of the subsequent test phases are in accordance with the circumstances in vivo. The applicability of the test devices and test procedures was successfully validated on numerous examples of clinical relevance as well as in the routine analyses of solid oral dosage forms.

